Discussion
Discussion
Huntington’s disease treatment options
Last updated Aug. 15, 2025, by Marisa Wexler, MS

Huntington’s disease is a genetic neurological disorder that’s marked by a variety of symptoms, including uncontrolled movements, cognitive difficulties, and mental health problems.
There is currently no cure for Huntington’s, and no medication can slow or stop the disease’s progression. However, there are several available Huntington’s disease treatment options that can help to ease symptoms and improve quality of life for people at all stages of Huntington’s disease.
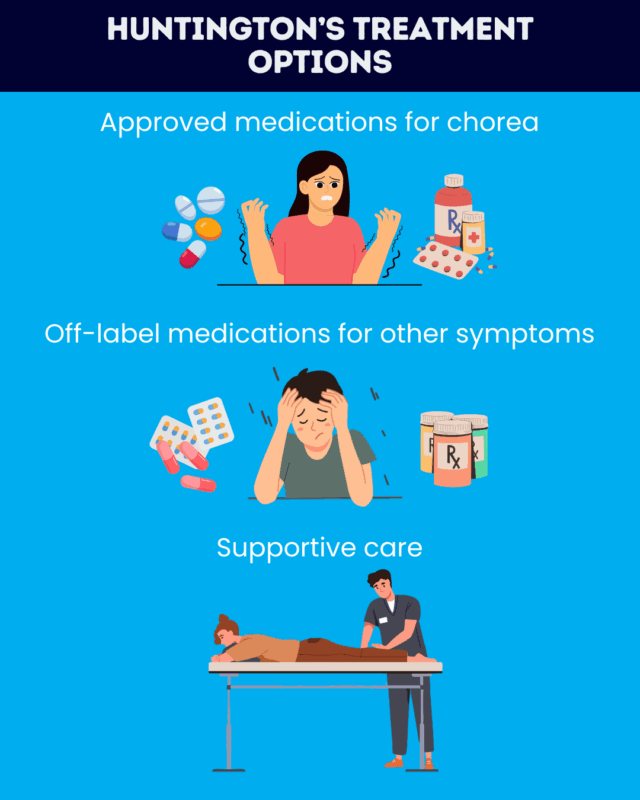
Approved treatments for Huntington’s disease
Currently, all the approved treatments for Huntington’s disease target chorea, a hallmark motor symptom marked by uncontrolled, irregular, dance-like movements.
Medications for chorea
Several Huntington’s disease medication options are available to help manage chorea, which can interfere with posture, walking, swallowing, and speech. In the U.S., the three daily oral medications approved for chorea treatment are:
- Xenazine (tetrabenazine)
- Austedo and Austedo XR (deutetrabenazine)
- Ingrezza and Ingrezza Sprinkle (valbenazine)
In the U.S., Austedo and Ingrezza are indicated specifically for adults, while Xenazine’s label has no age restrictions, but all three medications may be used for juvenile Huntington’s disease, which is marked by disease onset before age 20.
Off-label and symptomatic treatments
Besides the approved anti-chorea medications, several treatments are used to manage Huntington’s symptoms, especially its motor and psychiatric problems. There aren’t any treatments available to date that can delay or stop cognitive decline in Huntington’s disease.
Medications for motor symptoms
Some antipsychotic medications may be used to manage chorea, but they are not specifically indicated for this use. Other therapies also may be used off-label to help manage Huntington’s motor symptoms other than chorea.
For dystonia, a common Huntington’s symptom characterized by abnormal movements or postures due to excessive muscle contraction, muscle relaxants, as well as sedatives and anti-anxiety medications like benzodiazepines, may be prescribed.
Treatments that are approved for Parkinson’s disease may also be used to manage slowed movement, or bradykinesia, which is common in advanced stages of adult-onset Huntington’s and in the early stages of juvenile Huntington’s.
Treatment for psychiatric problems
Psychiatric symptoms of Huntington’s may be managed with the same types of medications that are used to treat mental health issues in the general population, such as antidepressants and antipsychotics, such as alprazolam and quetiapine. While not indicated specifically for Huntington’s, these therapies may be used as part of both adult-onset and juvenile Huntington’s disease treatment.
Specific treatment recommendations for managing psychiatric symptoms of Huntington’s include:
- Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs), two types of antidepressant medications for treating depression
- SSRIs and SNRIs for managing anxiety, but benzodiazepines like alprazolam (sold as Xanax, with generics available) may also be used
- SSRIs or stimulants for treating apathy.
- SSRIs for managing irritability and aggressiveness
- Sedative hypnotics or antidepressants for treating sleep disorders
- Atypical antipsychotics, a class of medications that includes quetiapine (sold as Seroquel, with generics available) for treating psychosis, that is, hallucinations and/or delusions
Supportive therapies and alternative treatments
In addition to medications, there are a variety of non-drug supportive treatments that may aid people with Huntington’s disease. These may include:
- physical therapy to improve strength and flexibility to maximize physical functioning
- occupational therapy to create strategies and adaptations, including the use of adaptive equipment, to navigate day-to-day life more easily
- speech therapy to help with communication as well as chewing and swallowing
- nutritional support to help ensure patients are getting the right nutrition to stay healthy
- psychotherapy, counseling, or other mental health support to help manage non-physical symptoms and cope with living with a life-threatening chronic disease
- palliative care to improve comfort and life quality for the patient and their family
- hospice care to help maximize comfort at the end of life
There has been some research into alternative treatments for Huntington’s, such as cannabis, acupuncture, nutritional supplements, or herbs used in traditional medicine practices. However, at present there is essentially no evidence that these alternative therapies are effective for helping manage Huntington’s symptoms.
Lifestyle changes
Changes in lifestyle cannot slow Huntington’s progression, but they can help patients maximize their functionality and overall health, which is important for maintaining a good life quality. Some lifestyle choices that may be beneficial for people with Huntington’s include:
- getting regular physical exercise
- eating a healthy diet that’s tailored to the patient’s individual needs
- maintaining consistent routines
- taking steps to manage stress, such as starting counseling
- maintaining good sleep hygiene
- making use of community resources such as support groups
Experimental treatments for Huntington’s disease
A range of different investigational therapies are in development for Huntington’s, either to slow or stop disease progression or to manage specific Huntington’s disease symptoms.
Gene-silencing treatments
A number of companies are developing gene-silencing medications to slow or halt Huntington’s progression. This type of treatment targets the disease’s underlying cause, a mutation in the HTT gene that results in the production of a longer-than usual huntingtin protein that is prone to form toxic clumps.
The majority of these experimental candidates work by suppressing the activity of both the mutated and healthy forms of the gene. The most advanced ones include:
Other gene-silencing therapies, including WVE-003, are designed to specifically target the mutated form of the HTT gene.
Medications targeting other disease-associated processes
Other ways to slow or stop the progression of Huntington’s are also being tested. Some investigational treatments in development for Huntington’s target biological processes that are thought to contribute to the disease. The most advanced candidates include:
- Pridopidine, which has shown potential to lessen neuroinflammation, improve nerve cell health and energy production, and reduce a type of cellular damage implicated in Huntington’s
- ANX005, which suppresses an immune protein that is thought to contribute to neuroinflammation and loss of connections between nerve cells
- Pepinemab, which works by blocking the activity of a protein implicated in Huntington’s-associated neuroinflammation and neurodegeneration
Symptomatic treatments
Several other strategies are being developed to treat Huntington’s symptoms, including:
- SOM3355, an experimental medication for chorea
- Deep brain stimulation, a surgical procedure that has been evaluated as a potential treatment of Huntington’s motor, cognitive, and psychiatric symptoms
Huntington’s Disease News is strictly a news and information website about the disease. It does not provide medical advice, diagnosis or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
Recent Posts
- Toward a better understanding of anger as a symptom of Huntington’s disease
- Actor Will Forte shares family story in Teva awareness campaign
- Finding ‘space in the middle’ to deal with life’s challenges
- What a compassionate reset looks like in life with Huntington’s
- Ingrezza engages with therapeutic target more strongly than Austedo
- Anticipation of a possible new clinical trial has us holding on to hope
- What caregiver burnout is really about — and what you can do about it
- Oral Huntington’s treatment aims to slow disease progression
- Accepting help from my loved one with HD is a lesson in partnership
- Understanding how Huntington’s disease affects my cognition
Related articles
-
 Discussion
Discussion
-
-
-