Dosing Starts in SELECT-HD Trial That Seeks to Lower mHTT Levels

A Phase 1/2 trial evaluating Wave Life Science’s investigational therapy WVE-003 for Huntington’s disease has started dosing patients, the company has announced.
The SELECT-HD trial (NCT05032196), underway in clinical sites in Australia, Germany, Poland, and the U.K., is currently looking to enroll 36 Huntington’s patients, ages 25 to 60, at the early stages of the disease and with a specific single nucleotide polymorphism (SNP), called SNP3, in the mutated HTT gene. An SNP is a change in a single nucleotide, the building blocks of DNA.
According to estimates, 40% of adults with Huntington’s carry the SNP3.
The trial is scheduled to end in December 2022.
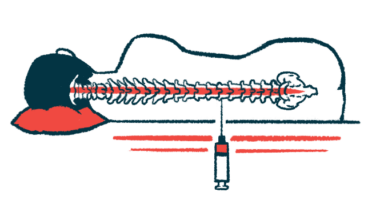
The study is testing the safety and tolerability of single- and multiple-ascending doses of WVE-003 versus a placebo, delivered via intrathecal injection, meaning directly into the spinal canal.
Additionally, it will assess the therapy’s pharmacokinetics — its movement into, through, and out of the body — and levels in the cerebrospinal fluid (which bathes the brain and spinal cord).
Other exploratory goals include its pharmacodynamics — the interactions between the body and a compound — among other clinical parameters.
During the trial, an independent committee will continuously guide the dosing regimen and frequency.
Huntington’s disease is caused by a mutation in the huntingtin (mHTT) gene, which leads to defects in huntingtin (mHTT) protein.
WVE-003 is an oligonucleotide designed to target the messenger RNA (mRNA) from the mHTT gene that carries the SNP3. mRNA is the molecule derived from DNA that is used as a template for protein production.
By binding directly to the mutated mRNA, WVE-003 prevents it from delivering the instructions for the production of the mHTT protein, lowering its production. It spares the mRNA with the instructions for the healthy (wild-type) HTT protein, which plays an important role in normal nerve cell function. This is called an allele-selective approach.
Growing evidence suggests that preserving the function of the healthy HTT protein in Huntington’s patients is linked to favorable clinical outcomes.
WVE-003 was designed with Wave’s unique PN backbone chemistry modifications (PN chemistry), which enables the generation of more stable, mRNA-targeting molecules. Moreover, the chemical tweak was shown to enhance the oligonucleotides’ activity, selectivity, and durability.
In both in vitro (lab) and in vivo studies, WVE-003 reduced the levels of the mutated HTT mRNA in a dose-dependent manner. The reduction was sustained in the brain’s cortex and striatum — a brain region involved in voluntary movement control — in animal studies.
“WVE-003 reflects the significant evolution of our chemistry and the many learnings gained from our first-generation clinical programs,” said Michael Panzara, MD, chief medical officer and head of therapeutics discovery and development at Wave Life Sciences.
“Our enthusiasm for this program is bolstered by a compelling set of preclinical data that demonstrated selectivity, potency, and durability of WVE-003 with effects in relevant brain regions,” he added. “Further, emerging data continue to indicate that a fundamental requirement for clinical success in [Huntington’s disease] treatment will be the need to preserve wild-type HTT protein, supporting our allele-selective approach to mutant HTT protein reduction.”
The SELECT-HD trial design and WVE-003 preclinical data were recently presented at the European Huntington’s Disease Network 2021 Remote Meeting, held Sept. 9–11.