New Method Allows The Study and Identification of Proteins in Brain Cell–Cell Interactions
Written by |
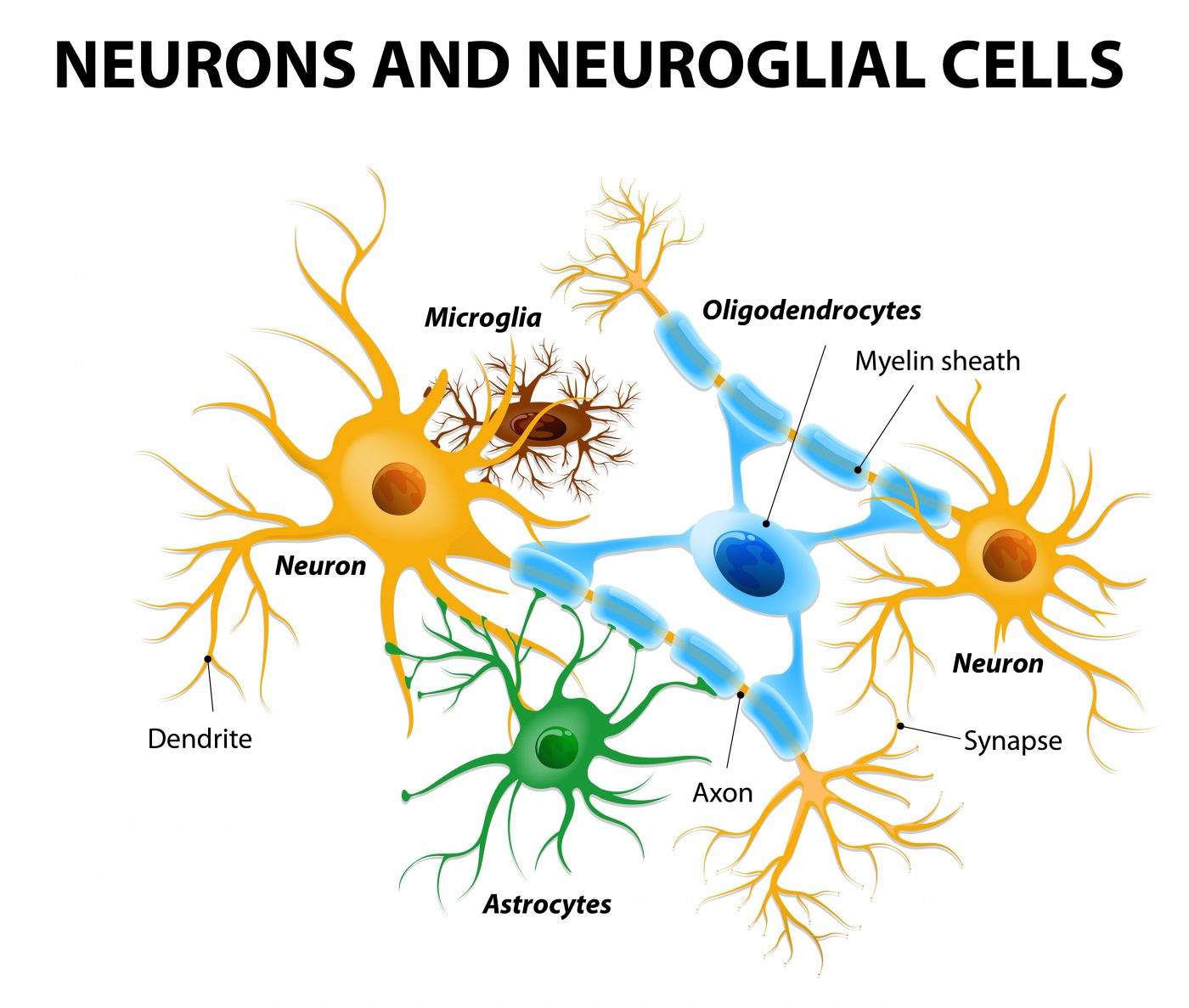
In a new study entitled “Spatial mapping of juxtacrine axo-glial interactions identifies novel molecules in peripheral myelination” a team of scientists reports a new method for capturing and understanding the molecular mechanisms behind brain cellular interactions, a key event for a healthy and fully functional brain which may hold implications for Huntington’s disease. The study was published in the journal Nature Communications.
The team identified novel molecules in brain cell–cell interactions that are necessary for the production of myelin – an insulating layer that forms around nerves in the brain and spinal cord and enables communication between nerve cells. The cellular interactions triggering myelin production are particularly challenging to identify, as Laura Feltri, MD, study lead author and an Hunter James Kelly Research Institute (HJKRI) researcher and professor of biochemistry and neurology in the Jacobs School of Medicine and Biomedical Sciences at Buffalo University noted, “Myelin is made by a glial cell wrapping around an axon cell. To study myelin, you really need to study both cells. The glial cell wraps like a spiral around the axon, so every time you try to study the region of contact between the two cells, you end up studying the whole combination. It’s very hard to look just at the interface.”
The team developed a new method that requires two chambers and a membrane to identify the interactions between neurons (specifically, axons, the long and slender projection of neurons that conduct electrical impulses) and glial cells. This novel technique relies on the ability of neurons to attract the glial cells, as Feltri explained, “When the cells in the upper chamber ‘recognize’ the cells in the bottom chamber, they kind of ‘reach’ through the holes in the membrane for each other and touch. That is the intersection that we can then isolate and study. Using this method, we can isolate the portion of a cell that comes in contact with another cell, and analyze all the proteins that are present only in this subcellular fraction. It provides a glimpse into the social life of cells.”
Using this technique,researchers identified new proteins in this glial-leading edge, including the prohibitins family of proteins, that upon deletion reduce axo-glial interactions and myelin production.
Feltri commented on the clinical implications of their findings, “This work has important implications for diseases of myelin such as Krabbe disease, and other neurodegenerative diseases, because the communication between glial cells and neurons is vital for neuroprotection.”
Yannick Poitelon, PhD, postdoctoral research scientist at Hunter James Kelly Research Institute (HJKRI) and study first author added, “This has profound implications for glial disease like Krabbe’s, Charcot-Marie Tooth, peripheral neuropathies or Multiple Sclerosis, because the dysfunction of glial cells end up impairing the interactions with neurons, which as a result suffer and degenerate causing devastating clinical symptoms. Similarly, neurodegenerative diseases like Huntington’s disease or Lou Gehrig’s, that were considered uniquely diseases of neurons in the past, are now considered diseases of cellular communications between neurons and glial cells.”


