Tiny Lipids as Delivery Vehicle May Make CRISPR-Cas9 More Effective Gene Editing Tool
Written by |
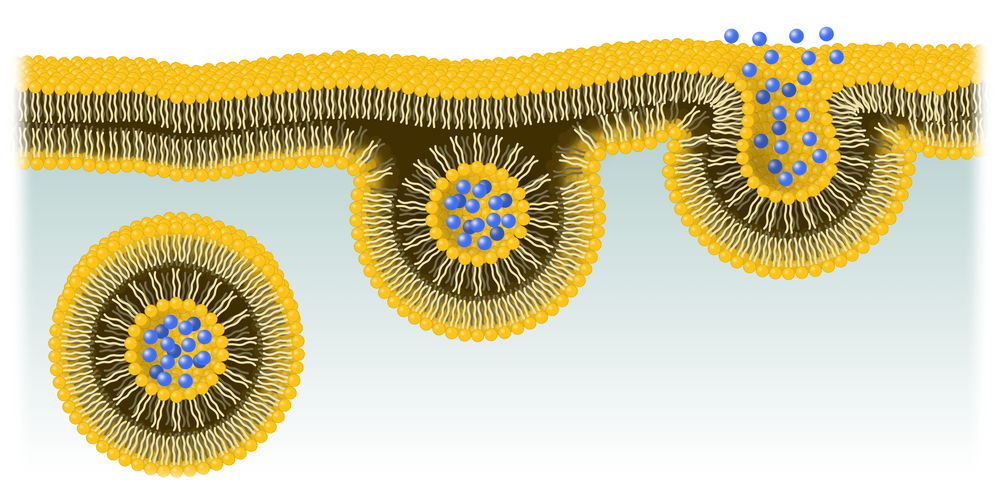
Tiny fatty (lipid) particles can be used to enhance the delivery of gene editing tools, such as CRISPR-Cas9, to targeted cells and considerably improve their ability to possibly treat human disorders like Huntington’s disease, researchers reported.
Their study, “Fast and Efficient CRISPR/Cas9 Genome Editing In Vivo Enabled by Bioreducible Lipid and Messenger RNA Nanoparticles,” was published in the journal Advanced Materials.
CRISPR-Cas9 is a gene editing strategy that uses enzymes to introduce breaks in specific DNA sequences. It is being explored as a way of inducing the destruction of faulty gene sequences or promoting their replacement to leave a healthy version of the targeted gene.
CRISPR technology has considerable potential applications, including altering the germline of humans, animals, and other organisms. By delivering the Cas9 enzyme and appropriate ribonucleic acids (RNAs) into a cell, an organism’s genome can be cut at any desired location, like a tailor-made gene modification.
In the case of Huntington’s disease, CRISPR-Cas9 can be used to remove the CAG (cytosine, adenine, and guanine) repeats in the coding sequence of the HTT gene, which are known to cause the disease.
To date, however, various attempts made to use this gene editing system to treat disorders have failed to show effectiveness with relevant clinical significance. To some extent, this has been due to limitations with systems, such as viral vectors, used to deliver and transfer all necessary CRISPR-Cas9 components into cells.
With this in mind, researchers have started to explore the potential of synthetic lipid nanoparticles (tiny fatty particles) as a delivery system.
Researchers at Tufts University and the Chinese Academy of Sciences engineered lipid nanoparticles that can be loaded with the Cas9 enzyme messenger RNA (mRNA) encoding sequence (much like an instructions manual) plus an engineered ‘single-guide’ RNA molecule (sgRNA). Of note, all genetic information contained within genes (DNA) needs to be transformed into mRNA before being ultimately translated into proteins.
These lipid nanoparticles can enter the cell where they are then disassembled, quickly and efficiently releasing their contents into the cell. Taking advantage of the cell’s natural machinery, the Cas9 enzyme will then be produced.
In the laboratory, this new system of simultaneous delivery of the Cas9 sequence and sgRNA molecule achieved up to 90% efficacy within 24 hours, “which is a significant enhancement” compared with other delivery systems, the researchers noted.
They also tested the efficacy of their new system in mice, seeking to lower the amount of the PCSK9 gene, which encodes a protein that regulates cholesterol levels in the bloodstream and can reduce the risk of cardiovascular disease.
The scientists were able to achieve a 20% reduction in PCSK9 protein levels in the blood in treated compared to untreated animals, using a single administration of nanoparticles loaded with Cas9/sgRNA. The nanoparticles were injected intravenously (through the vein), but traveled to and accumulated within liver cells.
“We can actually knock down PCSK9 expression in mice with 80 percent efficiency in the liver, suggesting a real promise for therapeutic applications,” Ming Wang, a co-senior study author and a professor at the Chinese Academy, said in a news release.
Collectively, these results “clearly demonstrated the high efficacy and biocompatibility” of this system for genome editing in living organisms.
“There are many diseases that have long been intractable for which CRISPR therapies could offer new hope — for example sickle cell disease, Duchenne muscular dystrophy, Huntington’s disease, and even many cancers,” said Qiaobing Xu, a co-senior author and an associate professor at Tufts. “Our hope is that this advance will take us another step toward making CRISPR an effective and practical approach to treatment.”


