Multiple dose findings in SELECT-HD trial of WVE-003 likely by June
Phase 1/2 study testing therapy targeting mutant huntingtin protein
Written by |
Note: This story was updated Jan. 24, 2024, to correct that Takeda is not supporting WVE-003’s development. Wave Sciences is the treatment’s sole developer; Takeda has a codevelopment and co-commercialization option.
New data from a Phase 1/2 trial of WVE-003, a therapy targeting the mutant huntingtin protein in people with Huntington’s disease, are expected in the coming months, according to an update from its developer, Wave Life Sciences.
Findings will include an extended follow-up of patients given multiple therapy doses, as well as a full dataset from those who received a single dose.
The SELECT-HD Phase 1b/2a clinical trial (NCT05032196) is recruiting up to 54 adults with early Huntington’s, ages 25-60 and with a particular disease-associated mutation, at sites in Australia, Canada, and Europe.
“We expect 2024 will be an inflection year that will drive significant value … most importantly, for the patients who will benefit from our research,” Paul Bolno, MD, president and CEO of Wave, said in a company press release. “These programs underscore our drive to ‘Reimagine Possible’ for science, for medicine, and for human health.”
WVE-003 aims to allow production of a healthy huntingtin protein
Upcoming trial findings, expected by June, will help Wave in making additional decisions about WVE-003’s clinical development, and those related to its ongoing collaboration with Takeda. Currently, Takeda has an option for a 50-50 codevelopment and co-commercialization of the investigational therapy.
Huntington’s is caused by mutations in the HTT gene, leading to production of an abnormal version of the huntingtin protein. While healthy, or wild-type, huntingtin (wtHTT) is critical for nerve cell health, the mutant form (mHTT) clumps in cells and turns toxic, driving damage and their dysfunction.
Wave believes that, in addition to the toxic effects of mHTT, the loss of wtHTT could contribute to disease symptoms. As such, WVE-003 is designed to specifically reduce production of the mHTT protein while maintaining wtHTT.
It does so by leveraging the fact that, for about 40% of patients, a mutation called SNP3 is found near the standard Huntington’s-causing defect on the mutated HTT gene.
WVE-003 is an oligonucleotide — a short strand of DNA or RNA — designed to bind to the place on HTT‘s messenger RNA where SNP3 resides. Messenger RNA is an intermediate molecule that serves as a template for producing a protein from DNA.
In binding to mutated messenger RNA, WVE-003 is able to prevent the template from being translated into the faulty protein. Because SNP3 will only be found on mutated messenger RNA, any messenger RNA containing instructions to produce wtHTT will be spared.
WVE-003 is “uniquely designed to lower mutant huntingtin and maintain healthy, wild-type huntingtin,” Bolno said.
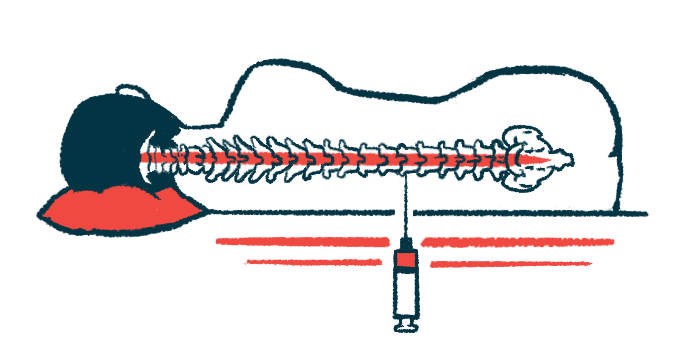
It is delivered via an injection directly into the fluid-filled spinal canal, called an intrathecal injection. In nonhuman primate studies, WVE-003 was effectively distributed in the brain after injection, according to Wave. Particularly, it was able to reach the striatum, a deep brain region that is especially affected in Huntington’s disease.
SELECT-HD is investigating WVE-003’s safety and pharmacological properties in early Huntington’s patients who have the SNP3 mutation.
In its single-dose part, patients were treated with a single intrathecal injection of WVE-003 at one of three doses (30, 60, or 90 mg) or given a placebo injection and monitored for about nine months (36 weeks).
Interim data shared last year showed that a single dose of WVE-003 (30 or 60 mg) led to a mean 35% reduction in mHTT in the cerebrospinal fluid — the fluid surrounding the brain and spinal cord — compared to a placebo. As expected based on its mechanism, wild-type HTT was preserved.
The therapy also was found to be safe and well-tolerated, with mostly mild or moderate side effects.
As these patients continue to be monitored, the safety, tolerability, and potential signs of efficacy of multiple 30 mg doses of WVE-003, delivered once every eight weeks, is being evaluated in another patient group.
SELECT-HD is expected to finish by the end of the year.