Targeting Astrocyte Brain Cells That Turn Toxic Could Help Treat Huntington’s, Other Diseases
Written by |
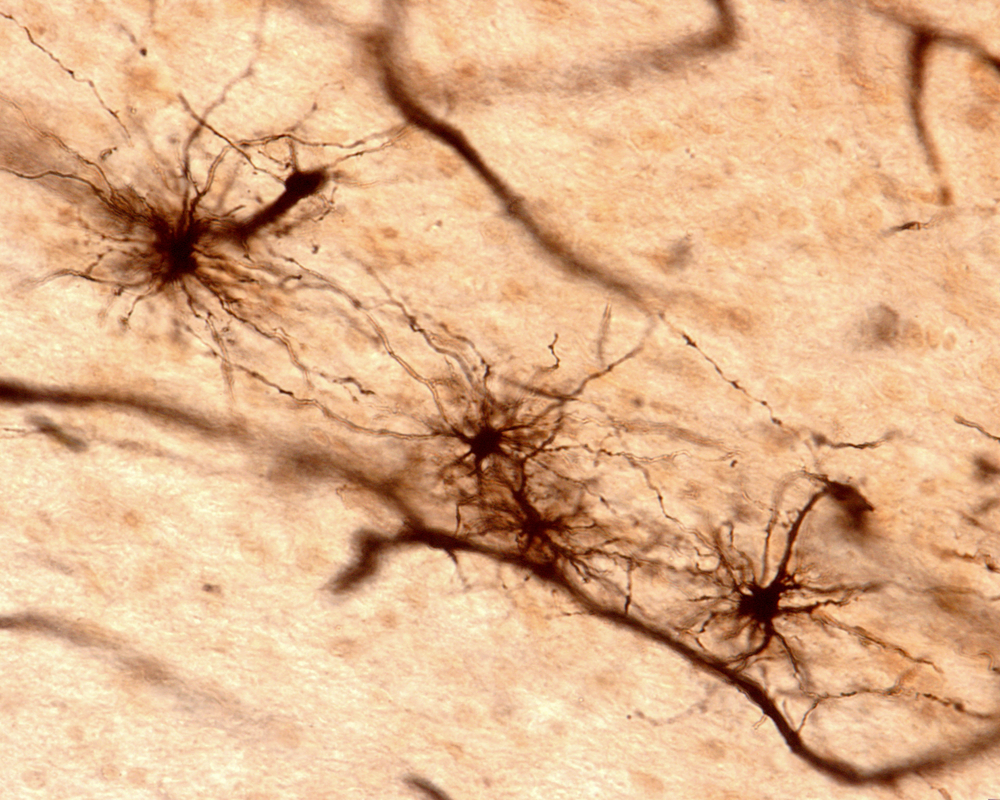
A brain cell subtype called astrocytes could be contributing to the death of neurons in neurodegenerative diseases such as Huntington’s disease (HD), according to a new study published in the scientific journal Nature.
This key finding, presented in a report titled “Neurotoxic reactive astrocytes are induced by activated microglia,” is important because it provides opportunities for scientists to develop new treatment approaches to treat Huntington’s and other neurodegenerative diseases.
Astrocytes are star-shaped cells found in the brain that are essential for the survival and healthy function of neurons. It is believed they are four times as abundant as neurons in the brain, and scientists know they could be transformed into what’s called “reactive astrocytes” as a result of brain injury, stroke, infection, or disease. But until now, it was not known whether these transformed astrocytes benefited or were detrimental to the nervous system.
“We’ve learned astrocytes aren’t always the good guys,” Dr. Ben Barres of the Department of Neurobiology at Stanford University School of Medicine, and the senior author of the study, said in a news release. “An aberrant version of them turns up in suspicious abundance in all the wrong places in brain-tissue samples from patients with brain injuries and major neurological disorders … The implications for treating these diseases are profound.”
In the past, the pharmaceutical industry mostly targeted neurons in an attempt to treat neurodegenerative disorders, Barres said. However, these diseases may be treatable by stopping astrocytes from turning into toxic cells that kill neurons.
Using a mouse model, the Stanford team of researchers showed that when exposed to LPS, a compound found on the surface of bacteria, the immune cells of the brain, called microglia, start secreting factors that induce inflammation such as TNF-alpha, IL-1-alpha, and C1q.
These pro-inflammatory factors turn resting astrocytes into what the researchers called A1 astrocytes, which lose the ability to promote the survival and growth of neurons and instead cause their death. Microglia can also secrete TNF-alpha, IL-1-alpha, and C1q as a result of neurodegenerative disease.
The researchers demonstrated this by using mouse retinal ganglion cells (RGC, cells that send information from the retina to vision-processing centers in the brain) grown in the laboratory. They added either resting astrocytes or A1 astrocytes to the cultured cells and saw that RGCs that were cultured in the presence of A1 astrocytes produced half the amount of synapses or connections compared to RGCs grown with resting astrocytes.
And they observed that the synapses that were formed in the presence of A1 astrocytes did not work as well. When they increased the concentration of A1 astrocytes, the researchers found that nearly all RGCs died.
The team also showed that A1 astrocytes were responsible for the death of neurons following injury. When they severed the optic nerve of the mice, the researchers saw that the A1 astrocytes quickly formed around the site of the injury. Interestingly, neutralizing the inflammatory factors TNF-alpha, IL-1-alpha, and C1q with antibodies prevented the formation of A1 astrocytes and RGC death in the mice, confirming that A1 astrocytes are indeed induced by these three factors.
In a final experiment, researchers analyzed brain samples from Huntington’s, Alzheimer’s and Parkinson’s disease as wells as ALS (amyotrophic lateral sclerosis) and multiple sclerosis (MS) patients and found that in each case, there were large numbers of A1 astrocytes clustered around the most active disease areas.
“We’re very excited by the discovery of neurotoxic reactive astrocytes,” Barres said, “because our findings imply that acute injuries of the retina, brain and spinal cord and neurodegenerative diseases may all be much more highly treatable than has been thought.”





