Brain Stimulation in Huntington’s Disease Promising, According to Study
Written by |
Germany and UK-based researchers have found that a surgical implantation procedure known as pallidal deep brain stimulation was safe when placed into the brain for the reduction of movement problems in Huntington’s disease. The study could allow for more research examining the effectiveness of the technique in larger groups of people with the condition. The report entitled “A Prospective Pilot Trial for Pallidal Deep Brain Stimulation in Huntington’s Disease”, appeared in the journal Frontiers in Neurology.
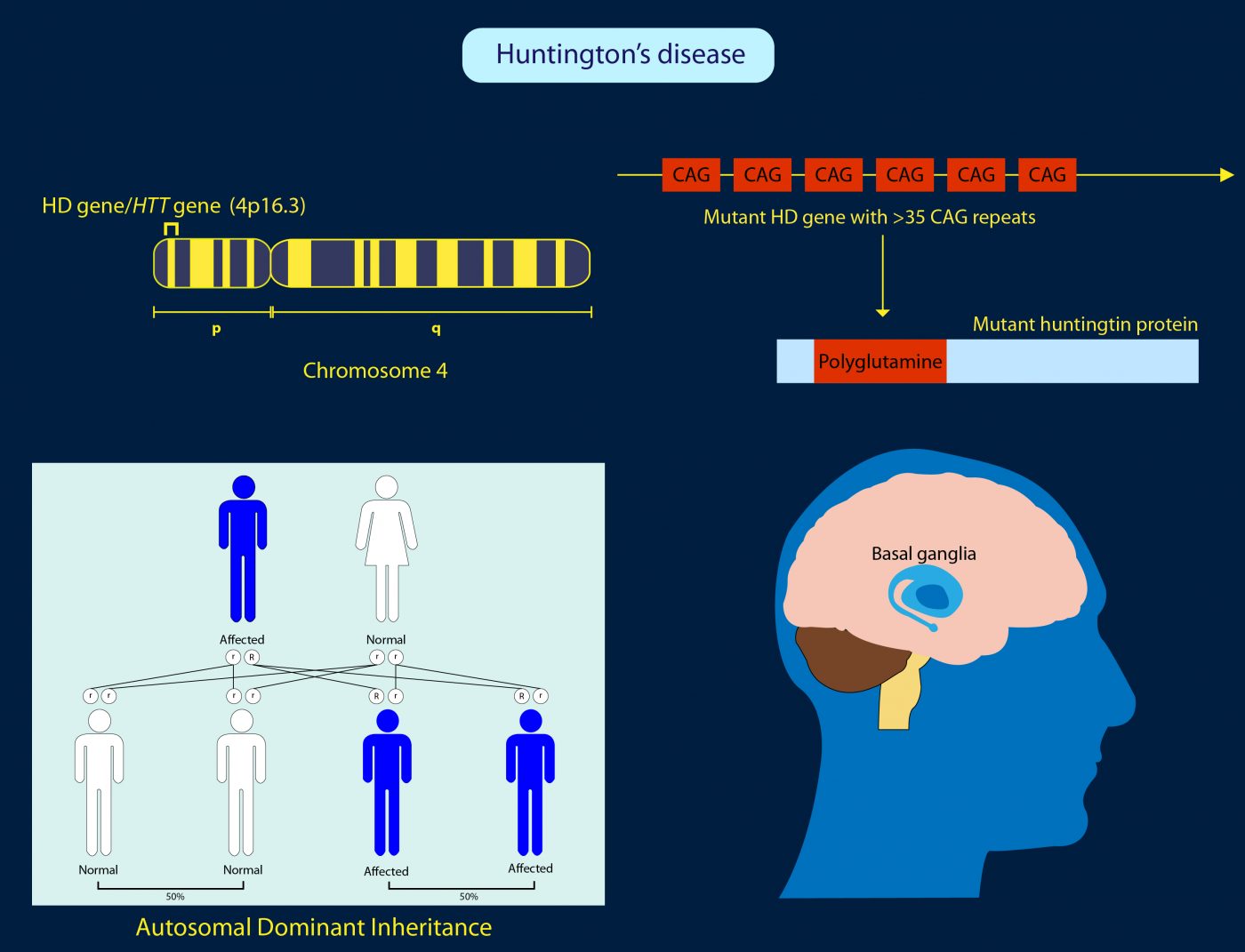
Huntington’s disease is a hereditary illness in which brain cells degenerate, causing involuntary, jerky movements known as chorea as well as dementia and psychiatric problems. It eventually leads to death. There is currently no cure and no effective treatments that can delay the disease.
Deep brain stimulation (DBS) involves the surgical implantation of an electrode in the brain. A stimulator is placed in the chest and wires are snaked through the neck to provide electrical stimulation to the brain. DBS has been used to treat depression and Parkinson’s disease, and may be effective for the treatment of Huntington’s disease.
Led by Lars Wojtecki of the Department of Neurology, Centre for Movement Disorders and Neuromodulation, Medical Faculty, Heinrich-Heine-University Düsseldorf, Germany, the investigators implanted electrodes into the globus pallidus of six people with Huntington’s disease, a region of the brain that controls movement and that is affected in Huntington’s disease. They measured the effectiveness of the stimulation using standard measurements for Huntington’s disease movement problems and examined how the treatment impacted cognition, mood, functionality/disability, and quality-of-life.
The scientists measured improvements in chorea, the involuntary jerking motions that are characteristic of Huntington’s disease. They did not observe improvements in dystonia, which is an abnormality of muscle tone that results in spasm and changes in posture. According to their report, they also found that mood and “some functionality/disability and quality-of-life scores improved significantly.” The treatment was generally found to be safe. The investigators noted eight adverse events, with two of them considered to be serious, however all resolved over time. There were no adverse events due to the surgery.
In their study report, the investigators state “Pallidal deep brain stimulation was demonstrated to be a safe treatment option for the reduction of chorea in Huntington’s disease. Their effects on chorea and dystonia and on quality-of-life should be examined in larger controlled trials.”
Because the trial only included a small number of study participants, more information about the effectiveness of this treatment needs to be obtained from larger studies containing more subjects. However, the research paves the way for further work examining a treatment for this destructive and life-debilitating disease.


