How Mutant Protein Drives Cellular Toxicity in Huntington’s Revealed in Study
Aggregates of mutant huntingtin protein have been associated with cellular toxicity in Huntington’s disease. Now researchers have discovered that isolated forms of mutated huntingtin are actually responsible for major molecular changes in cells that will drive toxicity.
The study, “Transcriptional profiles for distinct aggregation states of mutant Huntingtin exon 1 protein unmask new Huntington’s disease pathways,” was featured in the journal Molecular and Cellular Neuroscience.
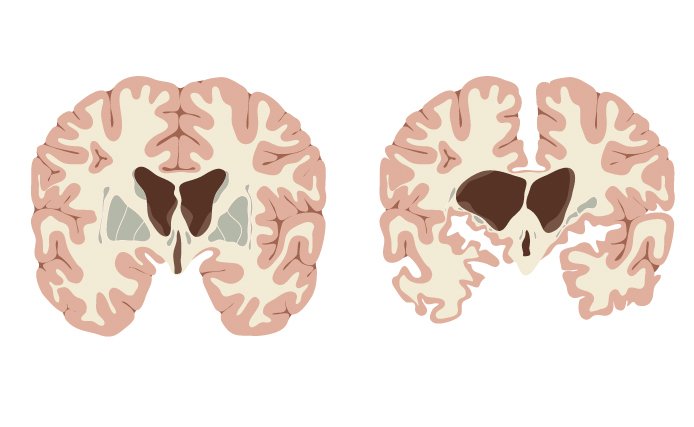
A code alteration in the gene that provides instructions for the huntingtin protein is the hallmark of Huntington’s disease. This genetic defect triggers the aggregation of these proteins, forming what are called amyloid fibrils and microscopic aggregates, also called inclusion bodies. Yet some studies have suggested that the problem arises before the formation of the inclusion bodies.
It was unclear how these aggregates contributed to disease progression or cellular toxicity. Adding to the biological complexity in Huntington’s disease is the fact that tissue samples collected from patients showed isolated modified proteins as well as aggregates at the same time, suggesting the possibility of different contributions.
Taking advantage of a method that could separate the aggregates from isolated forms of huntingtin, an Australian research team evaluated how these could influence the activity of cells. They found that, independent of the mutant huntingtin form present, the major change between Huntington disease cells and normal cells was driven by impaired signals triggered by CREB.
CREB is an essential transcription factor that is responsible for regulating the production of other proteins. Previous studies have implicated CREB deficiency in the development of HD. Still, in the present study, no significant changes were observed in the CREB mRNA sequence itself (the transcript from the gene) or on required CREB activators. All the changes were downstream of CREB and its signaling mediators.
This effect of mutant huntingtin was observed in cell lines with a human origin, but also in mice with Huntington’s disease.
A detailed analysis of the mechanisms affected by CREB dysregulation triggered by the mutant protein allowed the research team to identify four different regulatory signatures.
The first signature is invoked by the isolated mutant huntingtin that will generally promote the inhibition of the normal cells’ cleaning mechanism leading to the accumulation of cellular waste inside the cells. The second signature happens when inclusion bodies start to form that will promote toxic forms of proteins.
A third regulatory signature happens when the amount of aggregates in the cell increase, amplifying toxicity by the additive effect of both isolated and aggregate mutant proteins. And in the fourth signature, the signals of the isolated proteins will come to a normal basis and only those from the inclusion bodies will remain.
Together, this data suggest that the complex signaling system mediated CREB defects, driven by each of the forms of mutant huntingtin protein, is a central feature in Huntington’s disease.
“Our new roadmaps of the transcriptional [expression of proteins] changes associated with Htt [huntingtin] aggregation states offer a powerful resource to understanding how Httex1 [mutant huntingtin], notably the soluble [isolate] forms, propagates toxicity as well as in yielding CREB signaling as a therapeutic target for further investigation,” the researchers wrote.