Structure of Parkinson’s Disease Receptors Unveils Dual Role of Current Therapeutics
In a new study entitled “Allosteric interactions between agonists and antagonists within the adenosine A2A receptor-dopamine D2 receptor heterotetramer” researchers determined the structure of a key receptor underlying neurodegenerative diseases, including Parkinson’s disease, and showed how current drugs targeting these receptors and used to treat Parkinson’s can actually promote disease symptoms. The findings were published in the journal of the Proceedings of the National Academy of Sciences (PNAS).
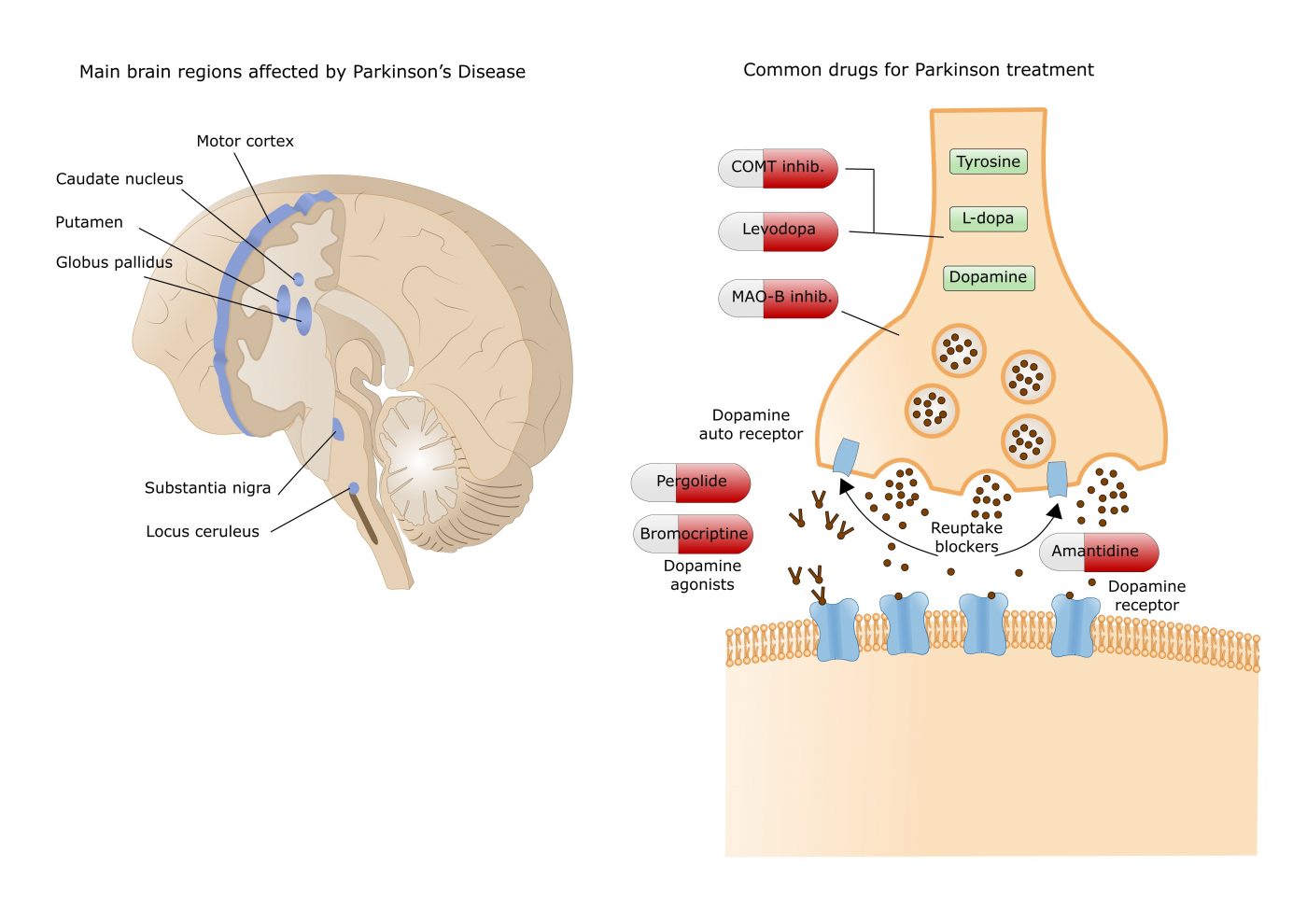
G protein-coupled receptors (GPCRs) are a big family of receptors sitting in the membrane of cells and have crucial functions in how cells communicate with each other. These receptors are quite complex, since they can exist as heteromers, i.e., composed of different subunits. In the brain, the GPCR-heteromer composed by adenosine A2A receptor (A2AR)-dopamine D2 receptor (D2R) regulates neuronal functions. Specifically, it can regulate the release of dopamine, a neurotransmitter that controls movement and emotional responses and is also involved in controlling the brain reward and pleasure centers.
Dopamine is the underlying trigger for several neurodegenerative diseases, including schizophrenia, Huntington’s and Parkinson’s disease. In fact, Parkinson’s disease symptoms are due to a deficiency in brain dopamine: when dopamine-producing nerve cells die, patients start developing motor problems such as tremor, slowness, stiffness and lack of balance.
While scientists already knew that adenosine A2A receptors bind dopamine B2 receptors, its structure remained unknown. Blocking adenosine A2A receptor in the brain, by using adenosine A2A receptor antagonists, was shown to reduce the effects of dopamine depletion, which ameliorate Parkinson’s disease motor deficits.
In this study, a team of researchers at the Department of Biochemistry and Molecular Biology of the University of Barcelona (UB), affiliated with the Centre for Networked Biomedical Research on Neurodegenerative Diseases (CIBERNED), determined that the structure of the adenosine A2A receptors bound to dopamine B2 receptors is a tetramer. This structure explains some odd results previously registered when administering antagonists of A2A receptors: while they are used to promote motor activation, they could also enhance motor inhibition. The tetrameric structure allowed researchers to understand that an antagonist drug of adenosine receptors A2A, such as caffeine, blocks adenosine-mediate inhibition of movement (adenosine is naturally being produce in our bodies). However, at higher doses, the antagonist may fail to block the effect of endogenous adenosine and actually behave like adenosine, activating the same signaling pathways. Hence, at these doses the antagonist behaves as an agonist and blocks dopamine D2 receptors.
The findings show the potential dual role of antagonists, as the authors noted, “The binding of receptors of hormones, neurotransmitters and neuromodulators forming tetrameters may explain the contradictory results obtained in therapies based on the use of an antagonist drug of a receptor to alter the action of the other receptor and underlines that the success of this type of treatments relies on administering an appropriate dose of the drug”.






